LABORATOIRES VIVACY
PIONEERS OF INTIMATE MEDICINE

OUR MISSION
TO HELP WOMEN LIVE BETTER EVERY DAY!
Still a taboo subject in society and too often ignored by the medical profession, vaginal dryness is nevertheless a public health problem that needs to be addressed collectively, given its physical and psychological impact.
Laboratoires VIVACY has taken an in-depth interest in women’s intimate problems to develop a therapeutic tool to improve the quality of life of women suffering from vaginal dryness during the pre-menopause and menopause, as well as patients undergoing treatment following cancer.
Alongside traditional therapeutic methods, the possibility of offering women a non-invasive treatment that is simple, fast and clinically proven to be effective represents a real medical advance in the treatment of intimate pathologies.
LABORATOIRES VIVACY
RENOWNED EXPERTS IN HYALURONIC ACID
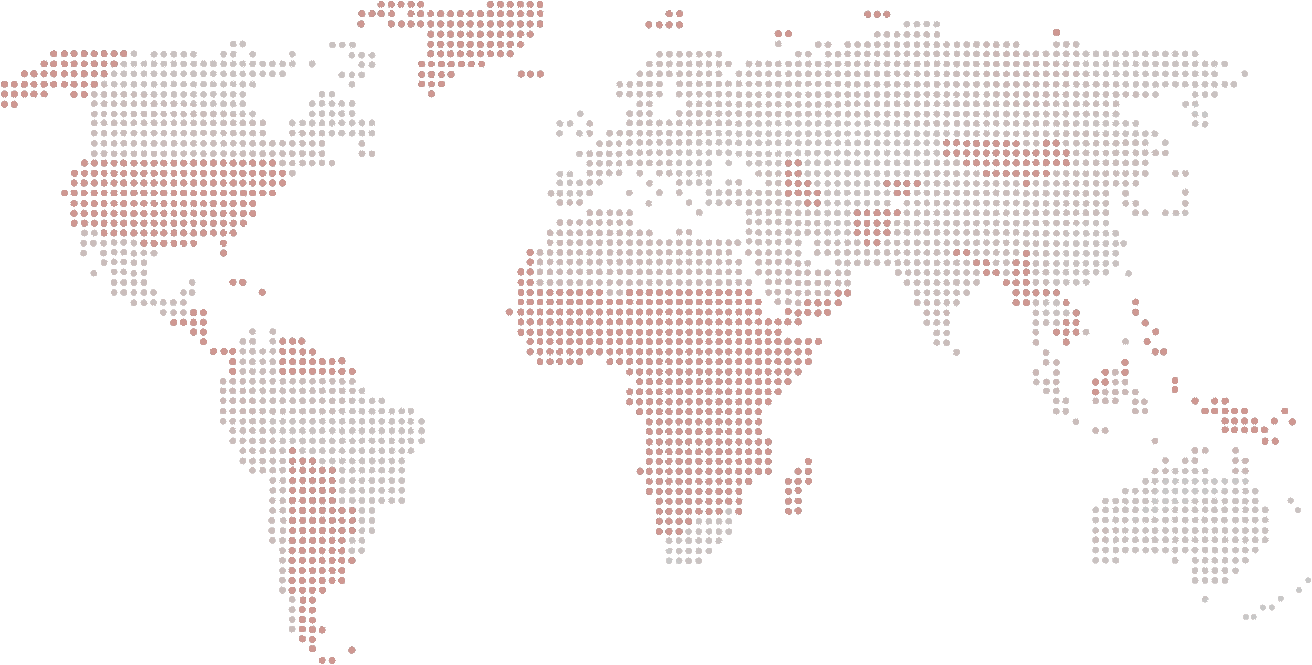
Today, Laboratoires VIVACY is a key player in the medical aesthetics and anti-ageing market. Founded in 2007, the French company specialises in the development, manufacture and distribution of innovative hyaluronic acid-based medical devices. VIVACY markets its products in France and in over 75 countries worldwide.
The Laboratoires VIVACY product portfolio comprises 3 main ranges: STYLAGE® range dedicated to facial aesthetics, DESIRIAL® range targeting gynaecological indications and a range of dermo-cosmetic products, VIVACY BEAUTY.
The company also manufactures hyaluronic acid gels for ophthalmic surgery, such as I-SPACE®, as well as viscosupplementation products for the treatment of pain in osteoarthritis of the knee, such as KARTILAGE®.
VIVACY manufactures its products in France, in Archamps in Haute-Savoie near the Swiss border, in its high-tech production unit, in strict compliance with European regulations on medical devices. All VIVACY products are manufactured in France.
1st injectable hyaluronic acid gel developed for women’s intimate health*
Designed and manufactured in France by Laboratoires VIVACY
Scientifically proven efficacy based on clinical studies

Our expertise at the service of your intimate health and well-being

Benchmark products on the intimate aesthetic medicine market for 8 years

distributed in 45 countries across the world
Follow us on instagram
*CE marking obtained in 2011.